Bond Parameters
Bond Parameters: Overview
This topic covers concepts, such as, Bond Parameters, Bond Length, Fajan's Rule & Application of Dipole Moment to Determine Percentage of Ionic Character etc.
Important Questions on Bond Parameters
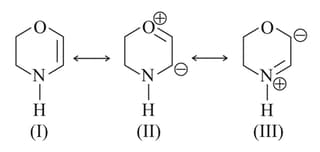
The least stable canonical structure among these is
For phenol, which of the following resonating structure is the most stable?
is more stable than because
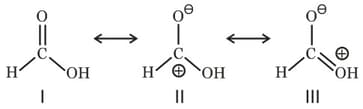
Among these canonical structures, the correct order of stability is
Among, these, which are canonical structures?
Bond formation is :
In the compound,  , the bond is of the type –
, the bond is of the type –
Which of the following compounds, hydrocarbons, has the lowest dipole moment?
A metal, M forms chlorides in its and Oxidation states. Which of the following statements about these chlorides is correct?
Based on lattice energy and other considerations which one of the following alkali metal chlorides is expected to have the highest melting point?
Which of the following compounds are dipolar?
Which of the following compounds is polar ?
Is it possible for the non-polar molecules to have polar bonds?
Explain why :
is more polar than bond.
Which of the following compounds are dipolar?
Amongst , the compounds with the greatest and the least ionic character, respectively, are.
Among the following, the most polar molecule is :
Polar covalent compounds ionize in their solution.
_____ covalent compounds dissolve in water.
Define polar and non-polar covalent compounds. Give an example of each.
